Cronobacter sakazakii in Powdered Infant Formula (PIF): a retrospective analysis
Late in December 2023, certain batches of a specialty powdered infant formula (PIF) product were recalled from the US market due to the potential contamination with Cronobacter sakazakii. This was a voluntary recall issued by the manufacturing company, after Israelian Health Authorities detected the presence of the bacteria in cans imported to Israel from the United States. The affected manufacturer performed extensive testing that only resulted in negative results, but due to the concerns raised, the company chose to issue a recall in the United States. Just a few weeks ago, in January 2024, the recall was expanded to include more distribution countries.
Announcements of this incident echo the events of 2022, when another voluntary recall by a different manufacturer of PIF products was issued in the United States. The FDA had received information about infant illnesses linked to consuming a certain PIF product, which initiated an onsite inspection in the manufacturing facility linked to those products and revealed insanitary conditions and the presence of Cronobacter sakazakii. In response to this, the FDA set out to follow a comprehensive strategy working with industry stakeholders to bolster the infant formula safety. Earlier in 2023, Cronobacter was also elevated to a nationally notifiable disease in the United States.
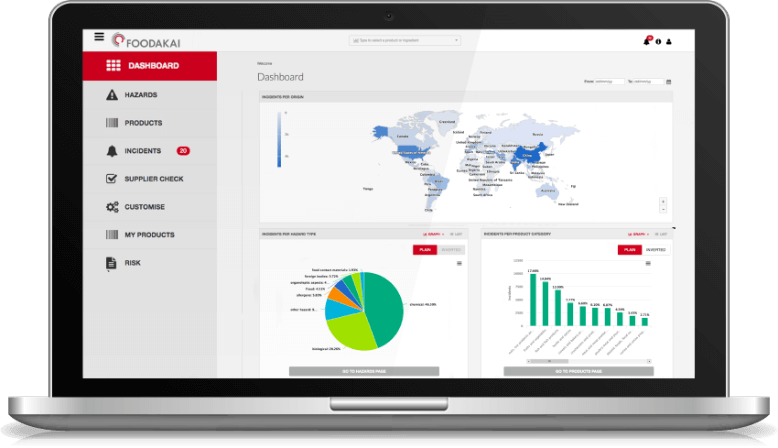
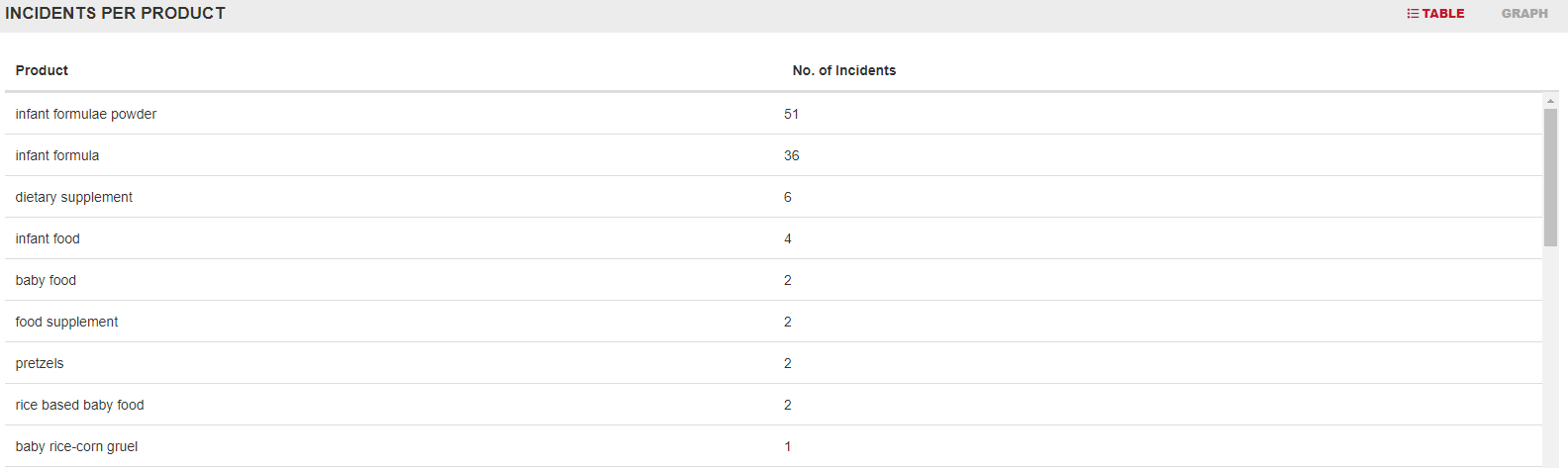
Historical Overview of Incidents Utilizing FOODAKAI, the food safety data analytics and AI-powered platform, it is evident that most incidents involving Cronobacter were reported in PIF and baby food products highlighting the vulnerability of these product categories to Cronobacter contamination.
Transmission & Health Issues related to Cronobacter sakazakii
In order to understand the reasons behind this association, an examination of the bacterial properties of Cronobacter sakazakii is necessary. These bacteria are found almost everywhere around us and are often introduced in food facilities on the soles of shoes or on hands and can live on surfaces like counters and utensils. This makes the pinpointing of a specific source of an incident extremely difficult but once again hygiene practices and staff training have come into the spotlight. Cronobacter also has the ability to survive in low moisture environments and this is one of the main reasons why it can be found in PIF products. Even though Cronobacter infections are rare (approximately 2-4 cases are annually reported to CDC), they could be life threatening in immunocompromised, premature and/or less than two-month-old infants, as this population is likely to develop meningitis if they get sick from Cronobacter.
Examining the trend of PIF Incidents
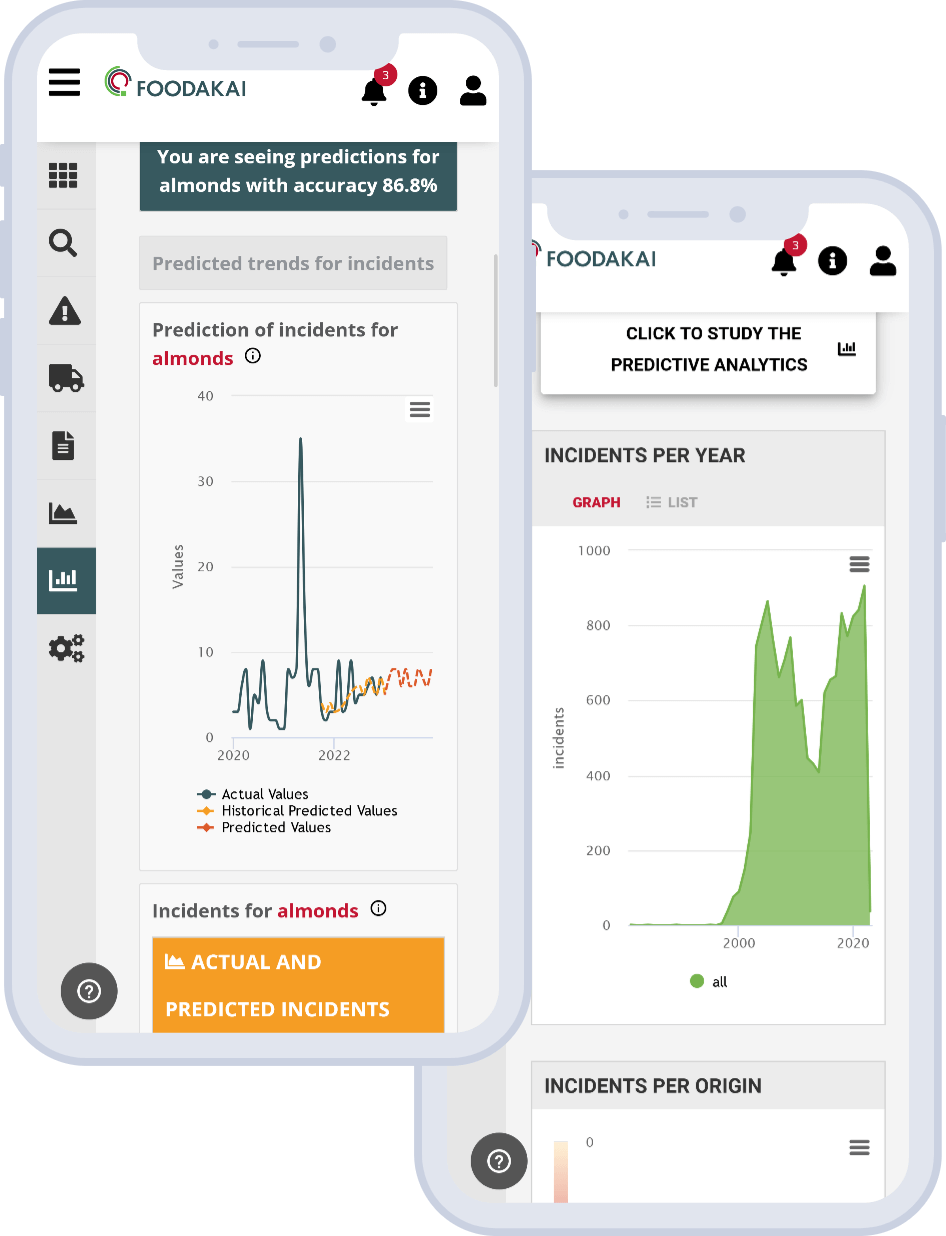
In order to better understand the trend it is necessary to examine past and future (forecasted) incidents linked to PIF, as captured in FOODAKAI.
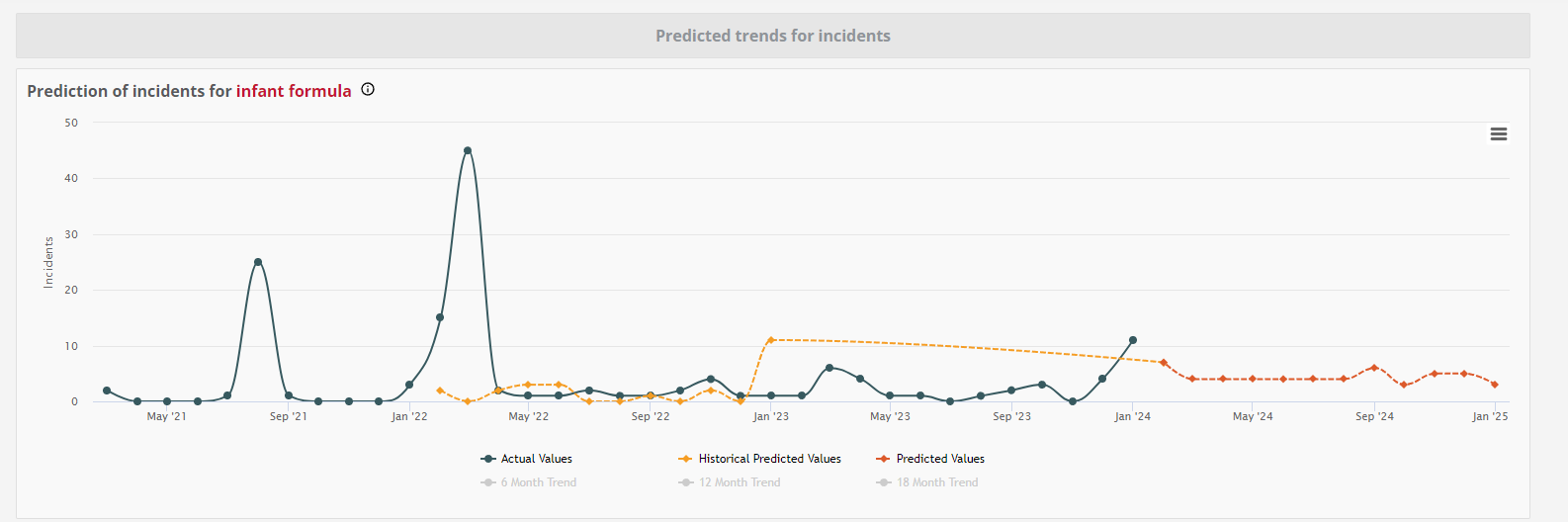
When examining this comprehensive visual summary, it is evident that since January 2023, the predicted values consistently surpassed the actual incident counts, projecting a concerning upward trend that could have served as an early warning to relevant food safety professionals. This trend continued up until January 2024, when the actual number of incidents surpasses the predictions.
While we could speculate that the expansion of the original 2023 recall to more countries where products had been distributed in 2024, may have influenced this, it aligns with the variability that can also been seen in past incidents and it shows the unpredictable nature of such incidents presenting an even greater challenge in anticipating them.
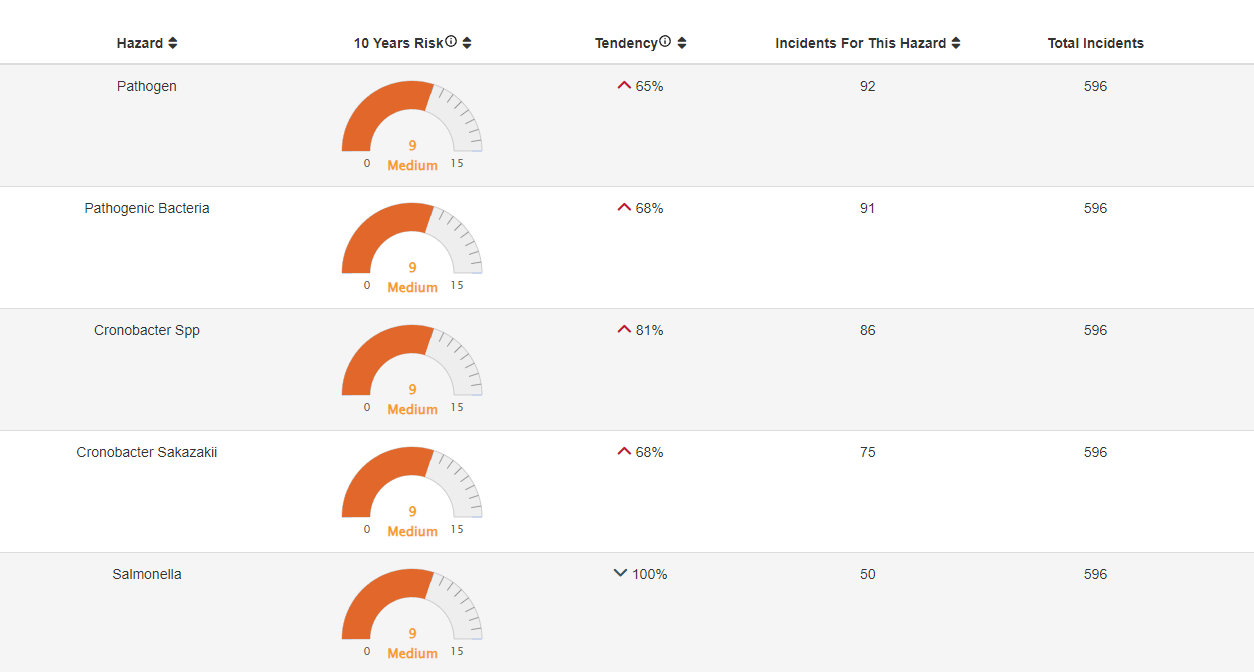
Applying FOODAKAI to the case Using the FOODAKAI platform, food safety professionals could have accessed the Hazard Analysis module to discover food safety risk insights on Infant formula. An alarming 81% increase in the tendency for hazards relating to Cronobacter is revealed and a 68% rise specifically concerning Cronobacter sakazakii.
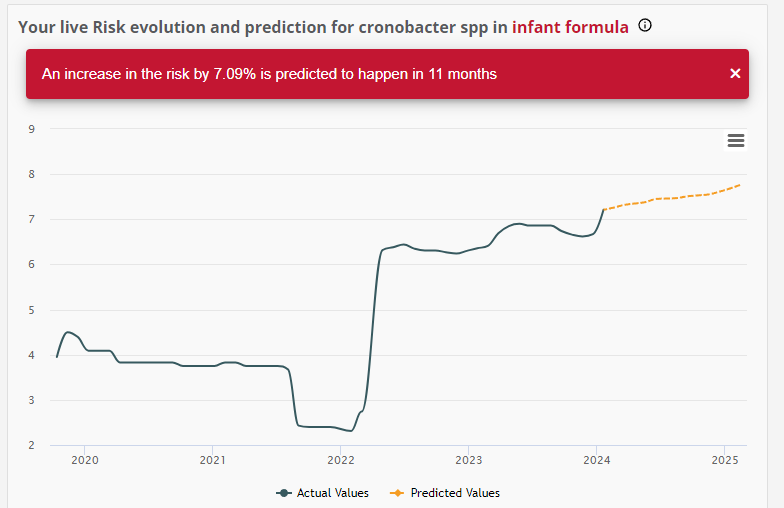
Focusing on the future to examine the risk evolution and prediction for the presence of Cronobacter in Infant Formula the findings are equally concerning: a 7% increase is predicted in the next 11 months.
Examining the events that unfolded in 2022 and in 2023, it is clear that relying solely on end-product testing is not sufficient to ensure the safety of PIF products. These past challenges highlight the significant need for a more proactive approach in order to safeguard products meant to be consumed by the most vulnerable category of consumers, infants.
FOODAKAI allows food safety professionals to benefit from early detection by accessing forecasts on incidents across all key ingredients and all hazard types. With a total view of where threat lies the AI-powered platform empowers proactive decision making, allowing food & beverage companies to move away from reactivity.
Learn more about how you can benefit from proactive risk prevention by scheduling a call. Schedule a call here.
Want to receive helpful food safety intelligence in your inbox?
 Funding for this research has been provided by the European Union’s Horizon Europe research and innovation programme EFRA (Grant Agreement Number 101093026). Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Commission-EU. Neither the European Union nor the granting authority can be held responsible for them.
Funding for this research has been provided by the European Union’s Horizon Europe research and innovation programme EFRA (Grant Agreement Number 101093026). Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Commission-EU. Neither the European Union nor the granting authority can be held responsible for them.